Implementing ISO/IEC 17025 Laboratory Management System: A Comprehensive Guide
ISO/IEC 17025 is an international standard that outlines the requirements for the competence of testing and calibration laboratories. Implementing this standard ensures that laboratories can produce valid and reliable results, leading to international recognition and customer confidence in their operations. For many organizations, using a readymade downloadable kit can significantly streamline the ISO/IEC 17025 implementation process by providing pre-designed templates, guidance, and resources.
This article provides a detailed guide to implementing ISO/IEC 17025 using this readymade kit, breaking down the process into manageable steps.
Understanding ISO/IEC 17025
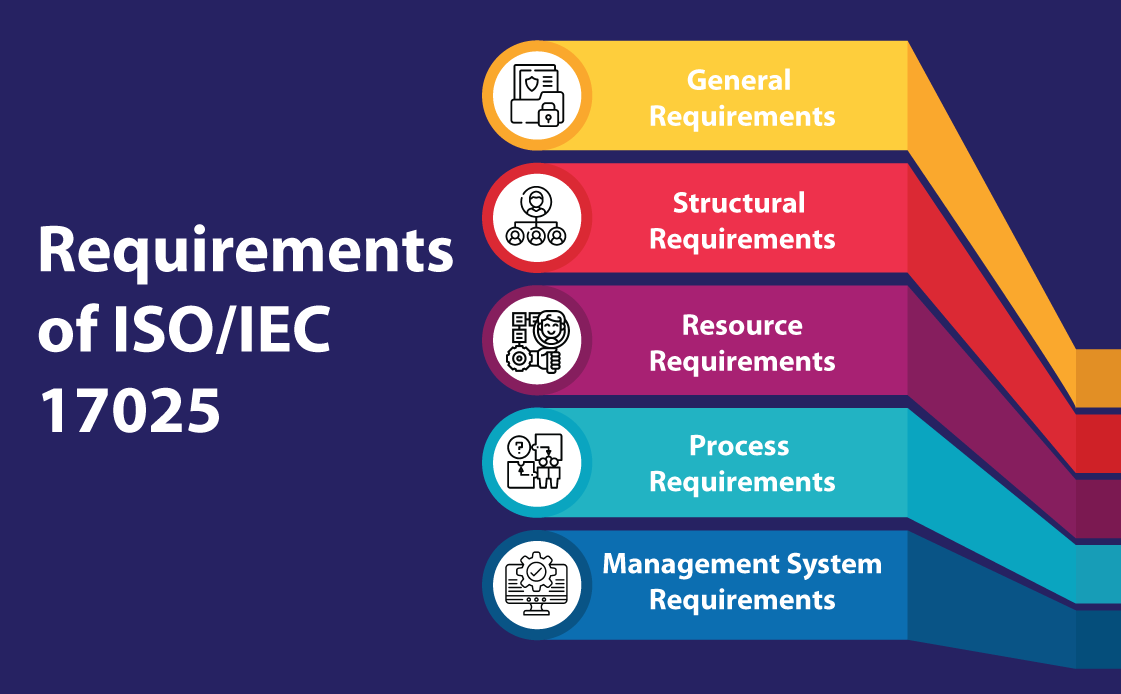
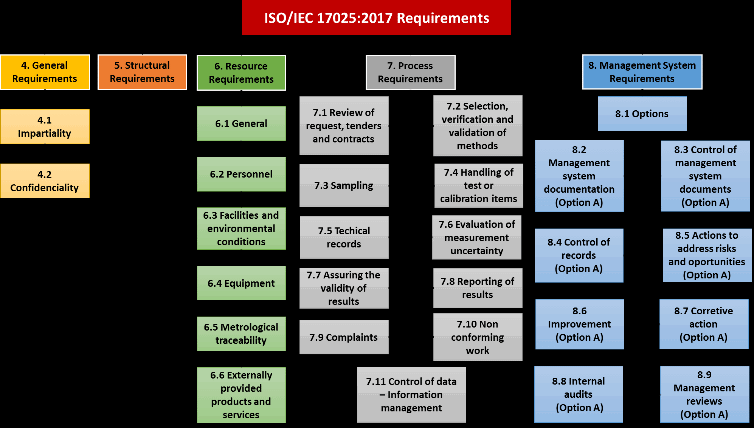
Before delving into the implementation, it is essential to understand the key components of ISO/IEC 17025:
- General Requirements: These include impartiality, confidentiality, and ethical behavior within the laboratory.
- Structural Requirements: The organizational structure, roles, and responsibilities.
- Resource Requirements: Involves the personnel, equipment, and infrastructure needed for testing and calibration.
- Process Requirements: The procedures followed for ensuring the validity of results.
- Management System Requirements: The documentation, records, and improvement processes necessary to manage laboratory activities effectively.
What is this Readymade Downloadable Kit?
The readymade downloadable kit is a comprehensive package of documents, templates, forms, and procedures designed to meet the requirements of ISO/IEC 17025. This kit include:
- Quality Manual: A template for developing a manual specific to the lab’s operations.
- Procedures and Work Instructions: Pre-drafted procedures for common lab activities.
- Forms and Templates: Documents like risk assessment templates, internal audit checklists, corrective action forms, and more.
- Training Materials: Guides to help train employees on ISO/IEC 17025 principles.
- Guidance Notes: Instructions on how to customize and use the templates effectively.
Click HERE to Download ISO/IEC 17025 Laboratory Management System Implementation Kit
Steps for Implementing ISO/IEC 17025 with a Readymade Kit
1. Obtain the Readymade Kit
The first step is to select a reliable vendor that offers an ISO/IEC 17025 implementation kit. Ensure the kit is up-to-date and covers all areas of the standard, particularly the 2017 revision, which introduced significant changes.
Click HERE to Download ISO/IEC 17025 Laboratory Management System Implementation Kit
2. Customize the Documentation
While the kit provides a solid foundation, you must tailor the documents to your lab’s specific operations. Here’s how to approach this:
- Quality Manual: Modify the provided template to reflect your lab’s structure, responsibilities, and processes.
- Procedures and Work Instructions: Adapt the general procedures to your laboratory’s specific equipment, materials, and methods.
- Forms and Templates: Ensure that forms, such as sampling records or calibration logs, align with your lab’s workflow and terminology.
Customization is crucial to ensure that the system genuinely reflects how your laboratory operates.
3. Conduct a Gap Analysis
A gap analysis helps you understand where your current processes fall short of ISO/IEC 17025 requirements. You can use checklists provided in the kit to compare your existing procedures with the standard’s criteria.
- Identify missing processes (e.g., documented risk assessment procedures).
- Determine whether existing processes need improvement or full redesign.
4. Develop Control of Data and Information Management Procedures
ISO/IEC 17025 places significant emphasis on the management of data and information, particularly in ensuring the integrity of results. This readymade kit include procedures for data control, which should be adapted to reflect your laboratory's systems and methods for handling test data, ensuring accuracy, security, and traceability.
Click HERE to Download ISO/IEC 17025 Laboratory Management System Implementation Kit
5. Establish Risk and Opportunities Management
A well-defined procedure for addressing risks and opportunities is key to ISO/IEC 17025 implementation. The readymade kit will often include a risk management procedure and risk register templates. Customize these to reflect your lab’s context by:
- Identifying potential risks (e.g., equipment failure, staff errors).
- Defining opportunities (e.g., process improvements, new technology).
- Documenting mitigation measures and actions.
6. Develop Sampling and Testing Procedures
Sampling and testing must be accurate and repeatable. Kits often include sampling plans and testing procedures that comply with ISO/IEC 17025 requirements. Customize these templates by integrating your specific methods for sampling and testing, considering factors like material type, environmental conditions, and measurement standards.
7. Implement the Documented System
With customized documentation in place, you now need to implement the procedures. Ensure:
- Employee Training: Staff should be familiar with the new processes and understand their roles in maintaining the quality system.
- Internal Communication: Hold regular meetings to discuss changes, address concerns, and ensure everyone understands the importance of ISO/IEC 17025 compliance.
Click HERE to Download ISO/IEC 17025 Laboratory Management System Implementation Kit
8. Conduct Internal Audits
Internal audits are a critical step in assessing the effectiveness of your management system before applying for certification. Use the audit checklists from the readymade kit to:
- Review your processes and ensure they align with documented procedures.
- Identify non-conformities and opportunities for improvement.
- Document corrective actions and track their implementation.
9. Management Review
Top management must review the system to ensure its continuing suitability and effectiveness. Use the management review templates to structure this process, covering:
- Results of internal audits.
- Customer feedback.
- Non-conformities and corrective actions.
- Performance against objectives and KPIs.
10. Apply for Accreditation
Once you are confident that your laboratory meets all the requirements of ISO/IEC 17025, you can proceed with the accreditation process. Accreditation bodies will conduct an external audit of your lab’s operations and management system. Ensure that all documentation is organized and accessible during this process.
Click HERE to Download ISO/IEC 17025 Laboratory Management System Implementation Kit
Benefits of Using a Readymade Kit for ISO/IEC 17025 Implementation
- Time-Saving: This readymade kit reduces the time needed to draft documents from scratch, allowing your team to focus on implementation.
- Cost-Effective: Purchasing this kit is often more affordable than hiring consultants for the entire process.
- Comprehensive Coverage: This kit include all required documents, ensuring no critical component is overlooked.
- Expert Guidance: This kit come with clear instructions, examples, and support to help you understand the implementation steps.
- Customization Flexibility: The templates are designed for easy customization, enabling you to tailor them to your lab’s specific requirements.
Click HERE to Download ISO/IEC 17025 Laboratory Management System Implementation Kit
Challenges and Tips for Success
- Customization Effort: While the kit provides a starting point, some efforts may be needed to ensure documents reflect actual practices. Allocate sufficient resources to this task.
- Employee Engagement: Ensure staff understand the changes being implemented and provide training to familiarize them with new procedures.
- Continual Improvement: ISO/IEC 17025 is not a one-time project. Regular audits, reviews, and updates are necessary to maintain compliance and drive continual improvement.
- Consult Experts When Needed: Even with a readymade kit, complex areas such as method validation, uncertainty analysis or employee training might require expert input. Don’t hesitate to seek additional help when necessary.
Conclusion
Implementing ISO/IEC 17025 using a readymade downloadable kit can significantly ease the process of achieving accreditation. By providing pre-designed templates, procedures, and forms, this kit allow laboratories to focus on tailoring the system to their specific needs, reducing the time and effort required to meet the standard's requirements. However, successful implementation requires thorough customization, staff training, and ongoing management commitment to ensure compliance and continual improvement.
Ultimately, a well-executed ISO/IEC 17025 implementation not only enhances a lab’s operational efficiency but also builds trust with customers and regulators by demonstrating a commitment to quality and accuracy.
Click HERE to download or any of the following documents:
ISO/IEC 17025 Laboratory Management System Implementation Kit
HACCP Implementation Kit
ISO 9001 Quality Management Systems (QMS) Implementation
ISO 22000 Food Safety Management Systems (FSMS) Implementation
Food Safety Systems Certification (FSSC) 22000 v5 Implementation
ISO 14001 Environmental Management Systems (EMS) Implementation
ISO 45001 Occupational Health & Safety Management Systems (OH&SMS) Implementation
ISO 50001 Energy Management Systems (EnMS) Implementation
Integrated Management Systems (IMS) Implementation
Production, Quality Control / Equipment Maintenance Kit
Lean Six Sigma
Lean Management/Manufacturing
Six Sigma Kit
Supplier Quality and Compliance Management (SQCM) Kit
Risk Management
Industrial Health, Safety & Environmental Management (HSE) Kit
Process Manuals