All-in-One Production Management Documentation Kit
Take control of your production operations with the All-in-One Production Management Documentation Kit—a powerful, downloadable collection of professionally designed templates tailored for efficient, compliant, and continuously improving production environments. Whether you're managing manufacturing, pharmaceuticals, food processing, or any other regulated production facility, this kit offers everything you need to streamline processes, uphold quality standards, and drive operational performance. Scroll down for details.....
Learn More
Take control of your production operations with the All-in-One Production Management Documentation Kit—a powerful, downloadable collection of professionally designed templates tailored for efficient, compliant, and continuously improving production environments. Whether you're managing manufacturing, pharmaceuticals, food processing, or any other regulated production facility, this kit offers everything you need to streamline processes, uphold quality standards, and drive operational performance.
📦 What’s Inside the Kit?
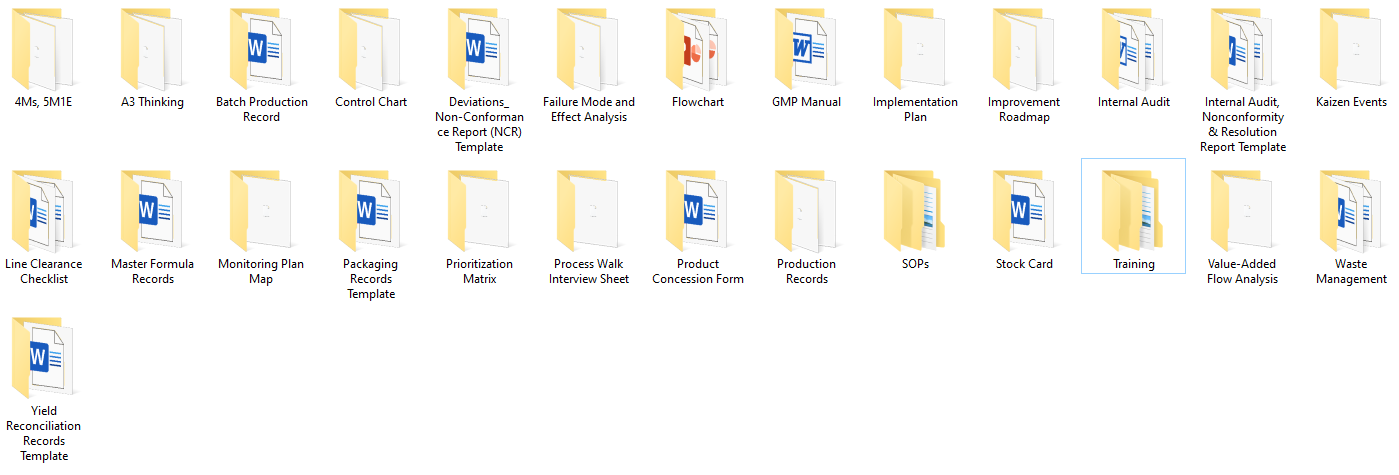
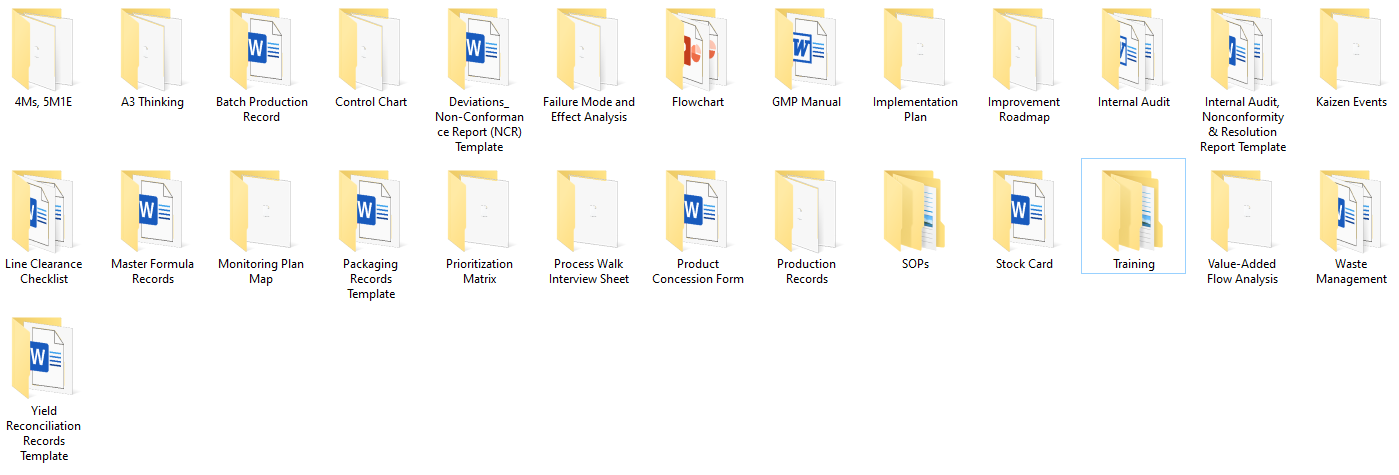
This comprehensive kit features 25+ high-impact, editable templates covering every key function in modern production management—planning, execution, quality, compliance, improvement, and training.
🏭 Production Operations Templates
Batch Production Record (BPR)
Ensure GMP compliance and full traceability of each production lot with structured batch record documentation.Master Formula Records
Maintain a standardized, validated recipe for each product to support consistency and regulatory compliance.Production Records (Various Types)
Track daily, weekly, and monthly production activities with ready-to-fill record templates adaptable to different operations.Line Clearance Checklist
Standardize production line readiness before and after batch processing to prevent cross-contamination and mix-ups.Packaging Records Template
Record packaging line operations, including materials used, output, and visual inspections.Stock Reconciliation Cards
Monitor raw material and finished goods inventory, ensuring accurate material accountability.
📊 Process & Quality Control Tools
4Ms and 5M1E Root Cause Analysis Templates
Use structured approaches for identifying and resolving production problems, covering Man, Machine, Material, Method, Measurement, and Environment.A3 Thinking Template
Facilitate lean thinking and structured problem-solving through this proven continuous improvement model.Control Chart Template
Visualize production trends, detect variances, and maintain statistical process control.Failure Mode and Effects Analysis (FMEA)
Anticipate risks, assess severity, and implement corrective actions to minimize production failures.Flowchart Template
Map processes clearly for training, improvement, and standardization efforts.Prioritization Matrix
Evaluate and rank improvement initiatives based on impact, feasibility, and urgency.
🛠️ Compliance & Risk Management
GMP Manual
Define good manufacturing practices and ensure operations meet regulatory requirements.Deviations & Non-Conformance Report (NCR) Template
Document any deviations from standard procedures and outline the corrective actions taken.Internal Audit Template
Plan and execute internal audits to evaluate compliance and operational readiness.Internal Audit, Nonconformity & Resolution Report Template
Capture findings, assign responsibility, and track resolution status.Product Concession Form
Manage conditional approval of non-conforming product use with documented justification.
🚀 Performance & Improvement Tools
Improvement Roadmap
Plan and track key improvement initiatives across departments or functions.Implementation Plan Template
Structure the deployment of new processes, tools, or compliance activities with timelines and responsibilities.Kaizen Event Template
Organize and document continuous improvement events with a focus on eliminating waste and boosting efficiency.Monitoring Plan Map
Visually track and manage critical process parameters, audit schedules, and improvement activities.Process Walk Interview Sheet
Gather insights directly from the floor by documenting staff observations and workflow assessments.
📘 Training & SOPs
Standard Operating Procedures (Various Types)
A robust collection of editable SOPs that can be adapted to match your production protocols and regulatory needs.Employee Training Record Template
Maintain detailed logs of training sessions, competency evaluations, and re-certifications.
🧩 Features:
Fully Editable – All documents provided in Word, Excel, or PDF formats, allowing easy customization for your organization.
Ready for Use – Save time on document development; start implementing best practices immediately.
GMP & ISO Friendly – Designed with compliance in mind to support standards like GMP, ISO 9001, ISO 13485, and others.
👥 Who Is This For?
Production Managers
Quality Assurance Teams
Compliance Officers
Continuous Improvement Leaders
Plant and Operations Managers
Download the Complete Production Management Documentation Kit today and take the guesswork out of documentation, compliance, and process improvement. Perfect for building a well-documented, audit-ready, and performance-driven production environment.