ISO/IEC 17025 Laboratory Management System Implementation Kit: Achieve Laboratory Accreditation with Ease!!
Are you looking to implement ISO/IEC 17025:2017 standards and streamline your laboratory operations for accreditation? Our ISO/IEC 17025 Implementation Kit is a comprehensive, ready-to-use solution designed to simplify your journey to compliance. Developed by industry experts, this kit provides you with everything you need to ensure your lab meets the highest international standards of testing and calibration quality. Scroll down for more details......
Learn More
Are you looking to implement ISO/IEC 17025:2017 standards and streamline your laboratory operations for accreditation? Our ISO/IEC 17025 Implementation Kit is a comprehensive, ready-to-use solution designed to simplify your journey to compliance. Developed by industry experts, this kit provides you with everything you need to ensure your lab meets the highest international standards of testing and calibration quality.
What’s Inside the Kit
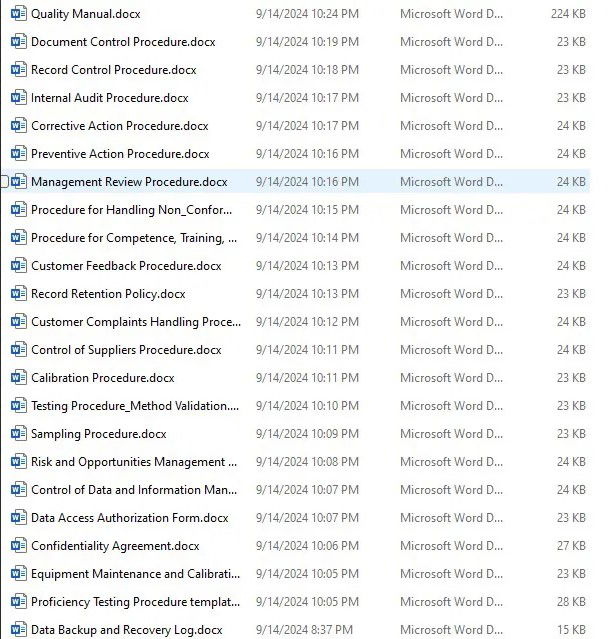
Quality Manual
Your foundation for ISO/IEC 17025 compliance, this manual provides clear guidance on quality management system policies, roles, and responsibilities within the laboratory.Document Control Procedure
Ensure seamless management, approval, and control of all critical documentation within your organization, keeping you audit-ready at all times.Record Control Procedure
A robust system for organizing, maintaining, and safeguarding records to ensure traceability and compliance.Internal Audit Procedure
Stay on top of your internal audits with a detailed procedure that ensures regular and thorough assessments of your compliance with ISO/IEC 17025.Corrective Action Procedure
React quickly and effectively to non-conformances with this step-by-step guide to identifying root causes and implementing lasting corrective actions.Preventive Action Procedure
Be proactive with our preventive action procedure, designed to help you identify potential issues before they impact quality and compliance.Management Review Procedure
Regularly evaluate the effectiveness of your quality management system with our comprehensive management review process.Procedure for Handling Non-conforming Work
Ensure the proper handling, review, and resolution of any non-conforming work in line with ISO/IEC 17025 requirements.Procedure for Competence, Training, and Awareness
Keep your team skilled, trained, and aware of their responsibilities with an organized and efficient approach to competence management.Customer Complaints Handling Procedure
Maintain customer satisfaction and improve laboratory services by effectively managing and resolving customer complaints.Control of Suppliers Procedure
Ensure that suppliers consistently meet your laboratory’s quality standards with this procedure for evaluating and monitoring supplier performance.Calibration Procedure
Maintain accuracy and precision in your laboratory’s measurement equipment with a thorough calibration procedure.Testing Procedure/Method Validation
Validate your testing methods and ensure compliance with industry standards, ensuring reliable and accurate results.Sampling Procedure
Define and standardize your sampling methods to guarantee the representativeness and reliability of results.Risk and Opportunities Procedure
A structured approach to identify, evaluate, and manage risks and opportunities that can affect your laboratory’s ability to deliver consistent results.Control of Data and Information Management System
Secure and manage your data efficiently with this procedure for ensuring the integrity and accuracy of all critical information.Equipment Maintenance and Calibration Records
Keep detailed and accurate records of equipment maintenance and calibration to maintain operational reliability and compliance.Proficiency Testing Procedure
Ensure your laboratory's ongoing performance and proficiency through external comparisons, validating the accuracy and reliability of your testing services.
Why Choose Our Kit?
- Fully Editable: Customize each document to meet the unique needs of your lab.
- Time-Saving: Cut down on preparation time and focus on implementation.
- Expertly Crafted: Created by industry professionals with extensive experience in ISO/IEC 17025 compliance.
- Audit-Ready: Be fully prepared for audits and accreditation assessments with meticulously structured documentation.
Who Is This Kit For?
- Testing and calibration laboratories seeking ISO/IEC 17025 accreditation.
- Laboratory managers looking to optimize operations and improve quality systems.
- Quality assurance professionals responsible for maintaining laboratory compliance.
Download the Kit Today!
Simplify your ISO/IEC 17025 implementation and achieve accreditation with confidence.
CLICK ON 'ADD TO CART!'
Start your journey toward excellence in testing and calibration with the ISO/IEC 17025 Implementation Kit today!