प्रयोगशाला प्रदर्शन को बढ़ाना: प्रयोगशाला प्रबंधन प्रणालियों (एलएमएस) में गुणवत्ता आश्वासन और नियंत्रण
Laboratories serve as the backbone of innovation and progress in scientific research, testing, and analysis, . However, the reliability and credibility of laboratory results heavily depend on the robustness of their management systems. Quality assurance (QA) and quality control (QC) play pivotal roles in ensuring the accuracy, precision, and integrity of laboratory operations. In this article, we delve into the significance of QA and QC in Laboratory Management Systems (LMS) and explore how they contribute to maintaining high standards of performance and reliability.
Understanding Laboratory Management Systems (LMS)
Laboratory Management Systems encompass a range of software and processes designed to streamline and optimize laboratory operations. These systems integrate various functions such as sample tracking, data management, instrument calibration, and compliance with regulatory standards. LMS aims to enhance efficiency, productivity, and data integrity within the laboratory environment.
Click Here to Download Readymade Editable Toolkits & Templates on Quality Assurance/Quality Control, Lean Six Sigma, Risk Management, Lean Manufacturing, Six Sigma, ISO 9001, ISO 14001, ISO 22000, ISO 45001, FSSC 22000, HSSE, Project Management etc.
Quality Assurance (QA) in LMS
QA in LMS focuses on establishing and maintaining processes to ensure that laboratory operations consistently meet predefined quality standards. It involves proactive measures to prevent errors, deviations, and inaccuracies throughout the entire testing and analysis workflow.
Key Components of QA
- Standard Operating Procedures (SOPs): Documented procedures detailing the steps to be followed for each analytical method, instrument operation, and data analysis. SOPs serve as guidelines for staff training, ensuring consistency and uniformity in laboratory practices.
- Training and Competency Assessment: Continuous training programs to equip laboratory personnel with the necessary skills and knowledge to perform their tasks accurately. Competency assessments help identify areas for improvement and ensure that staff members are proficient in their respective roles.
- Equipment Calibration and Maintenance: Regular calibration and maintenance of laboratory instruments to uphold accuracy and reliability in test results. Calibration protocols should be standardized and traceable to national or international measurement standards.
- Document Control: Effective management of documentation, including protocols, reports, and records, to ensure traceability, accessibility, and version control. Document control is essential for maintaining data integrity and facilitating regulatory compliance.
- Quality Audits and Inspections: Periodic internal and external audits to evaluate compliance with quality standards, identify areas of non-conformance, and implement corrective actions. Audits provide valuable feedback for process improvement and risk mitigation.
Click Here to Download Readymade Editable Toolkits & Templates on Quality Assurance/Quality Control, Lean Six Sigma, Risk Management, Lean Manufacturing, Six Sigma, ISO 9001, ISO 14001, ISO 22000, ISO 45001, FSSC 22000, HSSE, Project Management etc.
Quality Control (QC) in LMS
QC in LMS involves the systematic monitoring and evaluation of analytical processes and test results to detect and correct errors or deviations. Unlike QA, which focuses on preventive measures, QC is primarily concerned with detecting issues that may compromise the accuracy or reliability of laboratory data.
Click Here to Download Readymade Editable Toolkits & Templates on Quality Assurance/Quality Control, Lean Six Sigma, Risk Management, Lean Manufacturing, Six Sigma, ISO 9001, ISO 14001, ISO 22000, ISO 45001, FSSC 22000, HSSE, Project Management etc.
Key components of QC
- Analytical Method Validation: Validation of analytical methods to ensure their suitability for the intended purpose, including accuracy, precision, specificity, and robustness. Method validation confirms that the analytical results are reliable and reproducible within specified limits.
- Internal Quality Control (IQC): Ongoing monitoring of analytical performance using control samples with known concentrations or properties. IQC involves the regular analysis of control samples alongside patient or test samples to verify the accuracy and precision of test results.
- External Quality Assessment (EQA): Participation in proficiency testing programs administered by external organizations to assess the laboratory's performance relative to other laboratories. EQA provides an independent evaluation of analytical accuracy and identifies opportunities for improvement.
- Corrective and Preventive Actions (CAPA): Timely investigation and resolution of non-conformances, deviations, and incidents through CAPA processes. CAPA involves root cause analysis, corrective actions to address immediate issues, and preventive actions to prevent recurrence.
Click Here to Download Readymade Editable Toolkits & Templates on Quality Assurance/Quality Control, Lean Six Sigma, Risk Management, Lean Manufacturing, Six Sigma, ISO 9001, ISO 14001, ISO 22000, ISO 45001, FSSC 22000, HSSE, Project Management etc.
Conclusion
Quality Assurance and Control are indispensable components of Laboratory Management Systems, essential for ensuring the reliability, accuracy, and credibility of laboratory results. By implementing robust QA/QC processes, laboratories can uphold high standards of performance, meet regulatory requirements, and contribute to advancing scientific knowledge and innovation. Continuous improvement and adherence to best practices in QA/QC are imperative for maintaining excellence in laboratory operations and safeguarding public health and safety.
Click HERE to download or any of the following process improvement products:
HACCP Implementation Kit
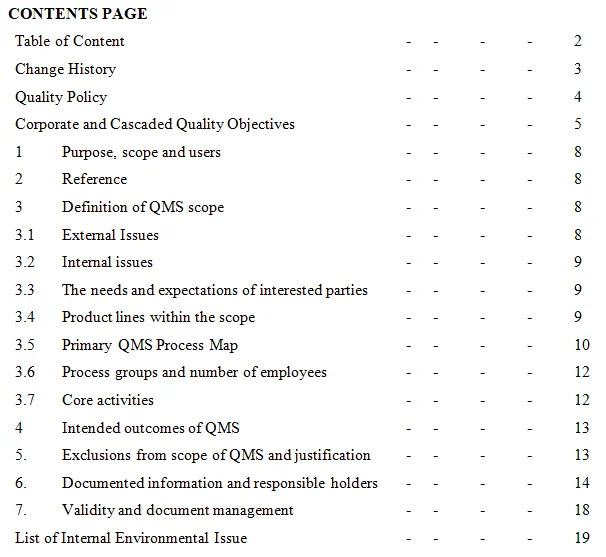
ISO 9001 Quality Management Systems (QMS) Implementation
ISO 22000 Food Safety Management Systems (FSMS) Implementation
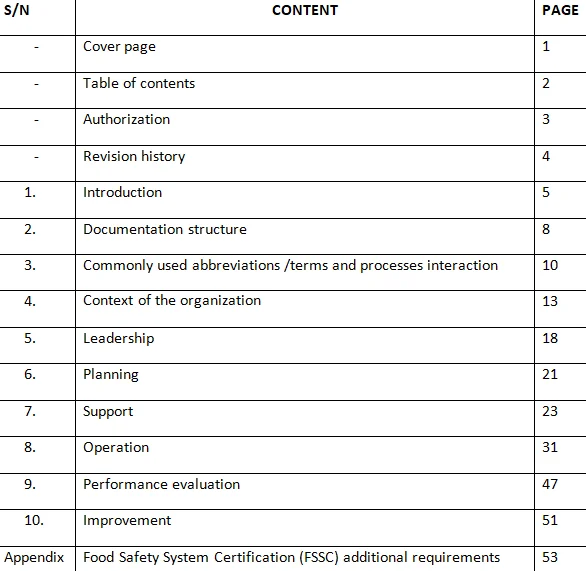
Food Safety Systems Certification (FSSC) 22000 v5 Implementation
ISO 14001 Environmental Management Systems (EMS) Implementation
ISO 45001 Occupational Health & Safety Management Systems (OH&SMS) Implementation
ISO 50001 Energy Management Systems (EnMS) Implementation
Integrated Management Systems (IMS) Implementation
Production, Quality Control / Equipment Maintenance Kit
Lean Six Sigma
Lean Management/Manufacturing
Six Sigma Kit
Supplier Quality and Compliance Management (SQCM) Kit
Risk Management
Industrial Health, Safety & Environmental Management (HSE) Kit
Process Manuals