आईएसओ/आईईसी 17025 - परीक्षण और अंशांकन प्रयोगशालाओं के लिए मानक निर्धारित करना
Quality assurance is key in every aspect of the industry. The reliability and accuracy of laboratory test results play a crucial role in various industries, including healthcare, environmental monitoring, manufacturing, and beyond. To ensure consistency and competence in testing and calibration laboratories worldwide, the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC) developed the ISO/IEC 17025 standard.
Understanding ISO/IEC 17025
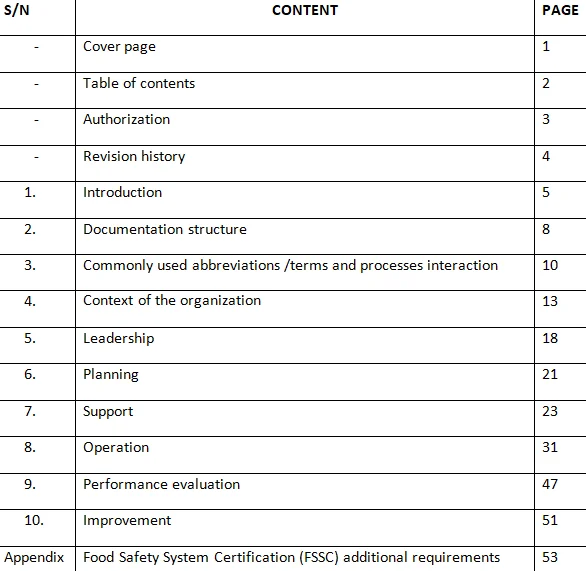
ISO/IEC 17025 lays down the general requirements for the competence of testing and calibration laboratories. It serves as a benchmark for laboratories to demonstrate their ability to deliver accurate, reliable, and consistent results. Whether it's testing the purity of pharmaceuticals, calibrating measuring instruments, or analyzing environmental samples, adherence to this standard ensures that laboratories operate with the highest level of quality management and technical competence.
Click Here to Download Readymade Editable Toolkits & Templates on Quality Assurance/Quality Control, Lean Six Sigma, Risk Management, Lean Manufacturing, Six Sigma, ISO 9001, ISO 14001, ISO 22000, ISO 45001, FSSC 22000, HSSE, Project Management etc.
Key Components of ISO/IEC 17025
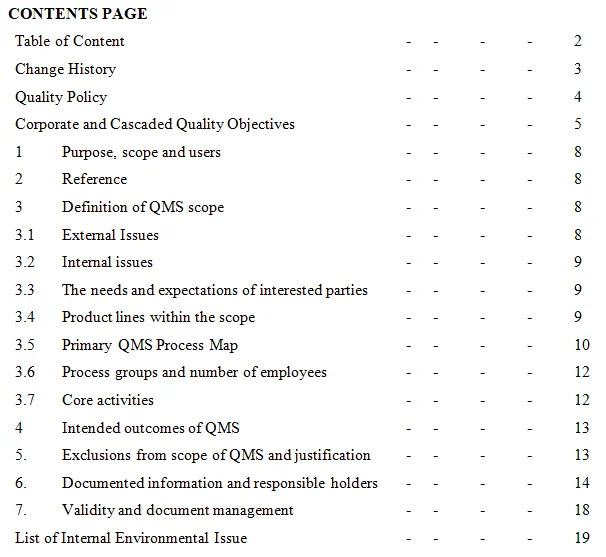
- Management Requirements: Laboratories must establish and maintain a quality management system to ensure the effectiveness and efficiency of their operations. This involves defining organizational roles, responsibilities, procedures, and policies to uphold quality standards.
- Resource Requirements: Adequate resources, including personnel, facilities, equipment, and materials, must be available to support laboratory activities. Competent staff, properly maintained equipment, and suitable testing environments are essential for accurate and reliable results.
- Process Requirements: Laboratories must follow standardized processes for sample handling, testing, calibration, and reporting. This includes ensuring the validity of test methods, the accuracy of measurements, and the traceability of calibration standards to internationally recognized references.
- Measurement Uncertainty: ISO/IEC 17025 emphasizes the need for laboratories to assess and report the uncertainty associated with their measurements. Understanding and quantifying uncertainty enables clients to make informed decisions based on the reliability of test results.
- Quality Control: Continuous monitoring and evaluation of laboratory performance are essential for maintaining quality standards. Laboratories must participate in proficiency testing programs, conduct internal audits, and implement corrective actions to address any identified non-conformities.
Click Here to Download Readymade Editable Toolkits & Templates on Quality Assurance/Quality Control, Lean Six Sigma, Risk Management, Lean Manufacturing, Six Sigma, ISO 9001, ISO 14001, ISO 22000, ISO 45001, FSSC 22000, HSSE, Project Management etc.
Benefits of ISO/IEC 17025 Certification
- Enhanced Credibility: Certification to ISO/IEC 17025 enhances the credibility and reputation of laboratories by demonstrating their commitment to quality and competence. Clients and stakeholders can have confidence in the reliability of test results.
- Global Recognition: ISO/IEC 17025 is recognized internationally, facilitating acceptance of test reports and calibration certificates across borders. This is particularly important for laboratories engaged in global trade and regulatory compliance.
- Improved Efficiency: Implementing standardized processes and quality management systems improves the efficiency of laboratory operations. Streamlined workflows, reduced errors, and optimized resource utilization contribute to overall productivity.
- Compliance with Regulations: Many regulatory bodies and accreditation agencies require compliance with ISO/IEC 17025 for accreditation of testing and calibration laboratories. Certification to this standard ensures alignment with regulatory requirements and industry best practices.
- Customer Confidence: ISO/IEC 17025 certification instills confidence in customers by providing assurance of the laboratory's technical competence and commitment to quality. It can be a significant differentiator in a competitive market landscape.
Click Here to Download Readymade Editable Toolkits & Templates on Quality Assurance/Quality Control, Lean Six Sigma, Risk Management, Lean Manufacturing, Six Sigma, ISO 9001, ISO 14001, ISO 22000, ISO 45001, FSSC 22000, HSSE, Project Management etc.
Conclusion
ISO/IEC 17025 serves as a cornerstone for ensuring the competence and reliability of testing and calibration laboratories worldwide. By adhering to this standard, laboratories demonstrate their commitment to quality, accuracy, and continuous improvement. Certification to ISO/IEC 17025 not only enhances the credibility and reputation of laboratories but also contributes to global harmonization and confidence in scientific and technical data. In an era where precision and reliability are paramount, ISO/IEC 17025 plays a pivotal role in upholding the highest standards of laboratory practice.
Click HERE to download or any of the following process improvement products:
HACCP Implementation Kit
ISO 9001 Quality Management Systems (QMS) Implementation
ISO 22000 Food Safety Management Systems (FSMS) Implementation
Food Safety Systems Certification (FSSC) 22000 v5 Implementation
ISO 14001 Environmental Management Systems (EMS) Implementation
ISO 45001 Occupational Health & Safety Management Systems (OH&SMS) Implementation
ISO 50001 Energy Management Systems (EnMS) Implementation
Integrated Management Systems (IMS) Implementation
Production, Quality Control / Equipment Maintenance Kit
Lean Six Sigma
Lean Management/Manufacturing
Six Sigma Kit
Supplier Quality and Compliance Management (SQCM) Kit
Risk Management
Industrial Health, Safety & Environmental Management (HSE) Kit
Process Manuals