अच्छे प्रयोगशाला अभ्यास (जीएलपी) के महत्व को समझना
Good Laboratory Practice (GLP) is a set of principles and guidelines established to ensure the quality and integrity of non-clinical laboratory studies. These standards are crucial for maintaining the reliability of data generated during experiments, which in turn supports the safety and efficacy assessments of various products, including pharmaceuticals, chemicals, agrochemicals, and biotechnological products. Adhering to GLP not only fosters trust in scientific research but also plays a pivotal role in regulatory compliance and decision-making processes.
What is GLP?
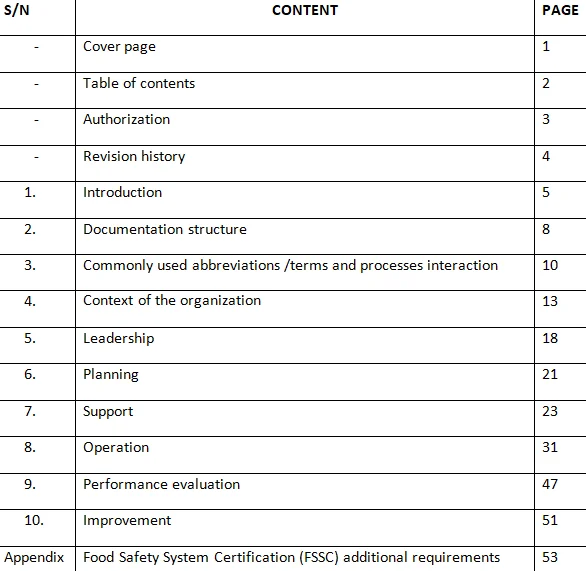
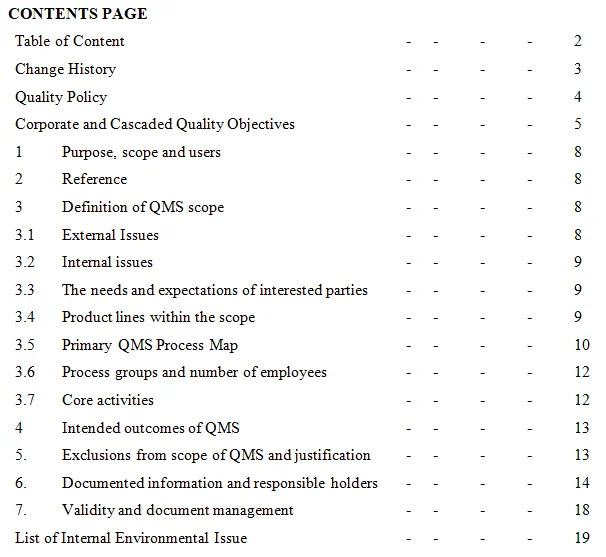
GLP encompasses a comprehensive framework of rules and procedures designed to promote consistency, reproducibility, and accuracy in laboratory experiments. It outlines the organizational structure, personnel qualifications, facilities, equipment, documentation, and operating procedures necessary to conduct studies in a controlled and reliable manner.Key
Click Here to Download Readymade Editable Toolkits & Templates on Quality Assurance/Quality Control, Lean Six Sigma, Risk Management, Lean Manufacturing, Six Sigma, ISO 9001, ISO 14001, ISO 22000, ISO 45001, FSSC 22000, HSSE, Project Management etc.
Components of GLP
- Facility and Equipment: GLP requires laboratories to maintain suitable facilities and equipment that meet predefined specifications. This includes controlled environmental conditions such as temperature, humidity, and lighting, as well as calibrated and validated instruments necessary for conducting experiments.
- Personnel Training and Qualification: Personnel involved in conducting GLP studies must possess appropriate education, training, and experience to perform their assigned tasks competently. Training programs should cover relevant protocols, standard operating procedures (SOPs), safety measures, and quality assurance practices.
- Standard Operating Procedures (SOPs): SOPs serve as detailed instructions for performing specific tasks within the laboratory. These documents outline the steps to be followed, including sample preparation, analytical techniques, data recording, and quality control measures. Adhering to SOPs ensures consistency and uniformity across different experiments and minimizes variability.
- Documentation and Recordkeeping: GLP emphasizes the importance of accurate and comprehensive documentation throughout the entire study process. This includes recording experimental procedures, observations, raw data, calculations, and any deviations from the protocol. Proper documentation facilitates traceability, auditability, and reproducibility of results.
- Quality Assurance (QA): QA measures are integral to GLP implementation and involve ongoing monitoring and evaluation of study processes to identify and address deviations, errors, or deficiencies. QA activities may include internal audits, inspections, proficiency testing, and corrective actions to maintain compliance with GLP standards.
Click Here to Download Readymade Editable Toolkits & Templates on Quality Assurance/Quality Control, Lean Six Sigma, Risk Management, Lean Manufacturing, Six Sigma, ISO 9001, ISO 14001, ISO 22000, ISO 45001, FSSC 22000, HSSE, Project Management etc.
Benefits of GLP
- Reliable Data: Adhering to GLP ensures the generation of high-quality, reliable data that can be used for decision-making purposes with confidence. Consistency in experimental procedures and documentation minimizes errors and enhances the reproducibility of results.
- Regulatory Compliance: Many regulatory authorities require GLP compliance for the submission of non-clinical safety data supporting product registrations or approvals. Meeting GLP standards facilitates regulatory acceptance of study findings and expedites the approval process.
- Cost-Efficiency: By promoting efficiency, consistency, and accuracy in laboratory operations, GLP helps to minimize resource wastage, rework, and potential delays in product development timelines. Investing in GLP upfront can lead to long-term cost savings and enhanced competitiveness in the market.
- Protection of Human Health and the Environment: GLP studies play a critical role in assessing the safety of various products, including pharmaceuticals, chemicals, and pesticides, thereby safeguarding human health and the environment. Rigorous testing conducted in accordance with GLP standards helps to identify potential hazards and risks associated with exposure to these substances.
Click Here to Download Readymade Editable Toolkits & Templates on Quality Assurance/Quality Control, Lean Six Sigma, Risk Management, Lean Manufacturing, Six Sigma, ISO 9001, ISO 14001, ISO 22000, ISO 45001, FSSC 22000, HSSE, Project Management etc.
Conclusion
Good Laboratory Practice (GLP) is an essential framework for ensuring the quality, integrity, and reliability of non-clinical laboratory studies. By adhering to GLP principles and guidelines, laboratories can generate data that is credible, reproducible, and compliant with regulatory requirements. Investing in GLP not only fosters trust in scientific research but also contributes to the protection of human health and the environment, ultimately supporting innovation and advancement in various industries.
Click HERE to download or any of the following process improvement products:
HACCP Implementation Kit
ISO 9001 Quality Management Systems (QMS) Implementation
ISO 22000 Food Safety Management Systems (FSMS) Implementation
Food Safety Systems Certification (FSSC) 22000 v5 Implementation
ISO 14001 Environmental Management Systems (EMS) Implementation
ISO 45001 Occupational Health & Safety Management Systems (OH&SMS) Implementation
ISO 50001 Energy Management Systems (EnMS) Implementation
Integrated Management Systems (IMS) Implementation
Production, Quality Control / Equipment Maintenance Kit
Lean Six Sigma
Lean Management/Manufacturing
Six Sigma Kit
Supplier Quality and Compliance Management (SQCM) Kit
Risk Management
Industrial Health, Safety & Environmental Management (HSE) Kit
Process Manuals